Streamlined DNA Extraction for Newborn Screening Workflows
Public health laboratories perform a broad range of services, but one of the most important — and often underappreciated — is newborn screening. This invaluable service helps catch potentially life-threatening conditions as early as possible for the best chance at positive outcomes for babies and their families.
On a technical level, newborn screening is very challenging. Scientists must run several screening tests from dried blood spot samples, which are known for their limited quantity and low quality.
To improve the yield of useful DNA from dried blood spots, Mei Baker and her team at the Wisconsin State Laboratory of Hygiene developed a higher-throughput and more efficient DNA extraction chemistry. Baker is known as one of the earliest pioneers in public health genetics, having introduced the world’s first newborn screening program for severe combined immunodeficiency (SCID) in addition to her extensive work with cystic fibrosis, fragile X, Pompe disease, and spinal muscular atrophy (SMA).
This DNA extraction technique provides such useful results that it was patented by the Wisconsin Alumni Research Foundation and is now licensed and sold as part of Quantabio’s Extracta™ DBS kit, which recovers PCR-ready genomic DNA in just 30 minutes, without the need for a purification step.
Meanwhile, the Baker lab has continued to adapt the approach for multiplex tests for SCID and SMA with Quantabio’s qPCR ToughMix reagents, engineered to withstand PCR inhibitors and provide accurate and robust results.
A Multiplexed Real-time Assay to Detect SCID and SMN1 Homozygous Exon 7 Deletion, and a Droplet Digital PCR Assay to Asses SMN2 Copy Numbers, in Newborn Screening for Spinal Muscular Atrophy - Baker et al., Poster presented at the APHL NBS 2019 Symposium. Read it here.
Other teams have developed assays for newborn screening for congenital cytomegalovirus (CMV) using the Extracta DBS kit.
We chatted with Baker to hear more about her DNA extraction technique. Here are a few highlights from our conversation.
Guided by a public health perspective
With a passion for public health science, Baker knew that any process she developed for DNA extraction had to meet specific criteria. “For these tests to be routine, we need more practical and simple approaches. I call it the need for ‘simple elegance,’” she says. “Simple elegance does not mean cheap, it means robust, easy-to-use, and dependable technology. A simple and elegant sample prep solution leads to better downstream application performance.”
One requirement in particular is the need to operate at scale. Public health labs process millions of samples annually, so they must have high-throughput options. “People usually think this means an automated liquid handling platform, but these instruments often face some difficulties associated with the physical nature of the dried blood spots,” Baker adds. “The robustness of Extracta DBS should help reduce or eliminate the need for these expensive robots.”
Working with dried blood spots
With the dried blood spot samples collected through newborn heel prick tests, any downstream analysis should be based on thoroughly vetted sample prep procedures. “A typical punch disk is 3.2 mm, which is equivalent to 3.2 μl of blood,” Baker notes. “In addition, amplifying small quantities of DNA could potentially lead to unwanted preferential amplification in PCR, and result in bias in subsequent data.”
Her team uses Quantabio qPCR ToughMix® reagents together with the Extracta DBS kit for optimal results. “I have tried several alternatives and the combination of Quantabio Extracta DBS and ToughMix reagent provided the best overall assay performance when we performed real-time PCR assays to measure T cell receptor excision circles used for SCID screening,” Baker says. “The extracted DNA has been successfully used for Sanger and next-generation sequencing assays.”
To see more of our conversation with Baker, check out this case study.
Future directions
As more newborn screening workflows make use of next-generation sequencing, the Quantabio team has focused product development efforts on making sure our reagents meet this demand. In a study from scientists at the U.S. Centers for Disease Control and Prevention, our Extracta DBS kit was successfully demonstrated in an NGS-based newborn screening program for cystic fibrosis.
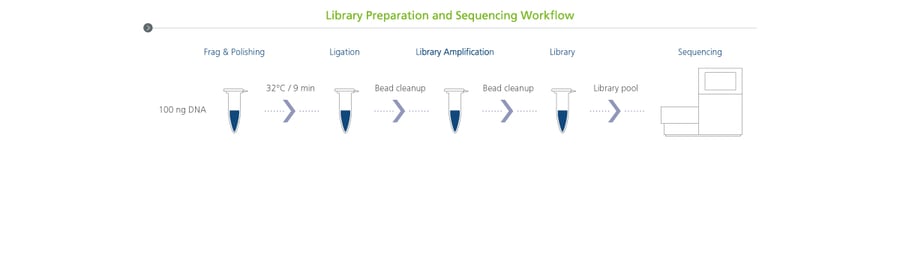
In addition, Quantabio’s suite of NGS products — sparQ DNA Frag & Library Prep, sparQ Universal Library Quant, and sparQ PureMag Beads — are all currently being evaluated to help expand testing menus, increase discovery rates, and minimize cost.
To learn more about our solutions for newborn screening, check out our dedicated application page
Recent posts


Subscribe to Our Blog
More Articles

Now Available: Step-by-Step Video Tutorials for sparQ NGS Products

Better QC for PCR-Free Libraries